Src tyrosine kinase
- a signaling protein that specializes in messages that control the growth of cells
- it relays its messages by adding phosphate groups to special tyrosine amino acids in protein chains. It adds phosphate groups to a wide variety of proteins that control cellular structure, cell communication, and cellular growth, turning them “on” and releasing them to perform their individual tasks
Redundancy in activity
- when one tyrosine kinase is blocked another one can pick up the function
- e.g. Abl kinase or Hck kinase do the Src function when Src is unavailable
Rouse sarcoma virus
researchers found that the Rous sarcoma virus, which causes tumors in chickens, injects a protein similar to the normal form of Src, called v-Src (for “viral Src”). v-Src, in contrast to the cellular c-Src, is always active. It continually adds phosphate groups to the many proteins serviced by Src, sending a constant, unwavering signal to grow. This leads to cancer, as cells grow without control into tumors.
Conformational flexibility
Explore oncogenic proteins
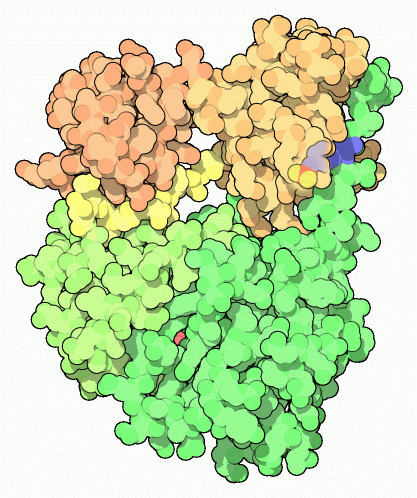
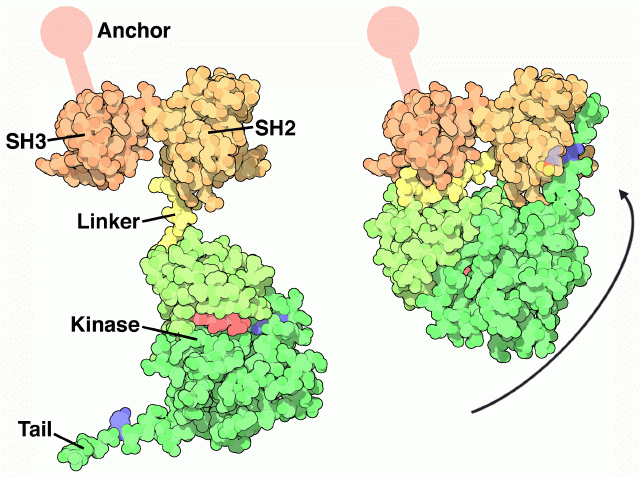
When exploring oncogene proteins, PDB entry 2src is a good place to start. This is the inactive form of Src, folded into a tight ball. The structure has a nucleotide bound in the kinase active site (shown in red here), and the key tyrosine (number 527 in the chain, seen here on the right side of the molecule) has a phosphate attached. You can also look at the Hck protein, PDB entry 2hck , which is almost identical to Src. A fragment of the Abl protein, which at full-length is much larger than Src and Hck, is also available in PDB entry 1opl . It is slightly different than Src and Hck, and doesn’t use a tail to stabilize the closed form. This structure includes a drug molecule in the active site (shown in red here) and a lipid bound in a deep pocket (shown in gray).