Cubanes and geometric algebra
The flat hexagon of a benzene is one of the first structures we encounter in organic chemistry and it stays with us for a while. If out of six positions on the ring two are different than the other four, we encounter first example of regioisomers; when substitents are adjacent we call this the ortho isomer, when there is one carbon between this is meta, and if they are two carbons away, para isomer. Since this entire system is in a plane, it’s fairly obvious to see that the angle between the substituents in the ortho isomer is $60^{\circ}$ degrees (or $ \pi/3 $), if they are meta it is $ 120 ^{\circ} $ or $ 2\pi/3 $ and if they are para, the angle is $180^{\circ}$ (or $ \pi $).
Now everyone wants to “escape the flatland” and chemists figured that there is some resemblance between the flat benzene and decidedly three-dimensional cubane. Recently, MacMillan and collaborators from Merck published a technique to derivatize cubanes and I wondered what is the angle between the adjacent positions in cubane, or the ones that are a face-diagonal away, or the ones that are the main diagonal away. You can see that if cubane is analogous to benzene and one of the verices is substituted, than there can be three “ortho” positions, 3 “meta” positions, and one “para” position. This is nice, but what is really the angle between these bonds? (Yes,the authors gave the numbers, but where do these numbers come from? Even if you have a model, the software may not have an easy way to measure the angle between nonadjacent directions.)
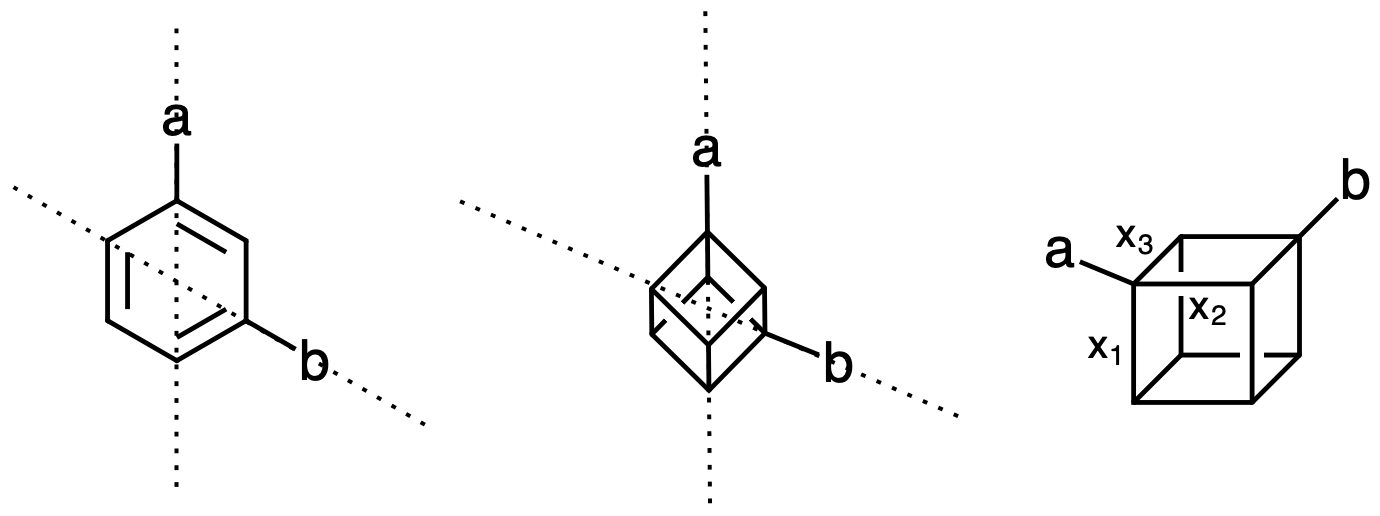
Luckily, this can be easily done with some simple geometric algebra, or in this case just the scalar product between vectors will do. Thus we define:
\[a = \frac{-x_1-x_2-x_3}{\sqrt{3}}\]and
\[b = \frac{-x_1+x_2+x_3}{\sqrt{3}}\]and then
\[a \cdot b = ||a|| ||b|| \cos{\theta} = -1/3\]And the angle between a and b is $ 109.47^{\circ} $, which we know as the angle of a tetrahedral carbon. This is unsurprising if we remember that the tetrahedron can be inscribed in a cube exactly by connecting the four vertices with the diagonals of the faces.
For the quasi-ortho position, keeping the vector $a$ constant, we can have:
\[b = \frac{-x_1+x_2-x_3}{\sqrt{3}}\]and this gives:
\[a \cdot b = ||a|| ||b|| \cos{\theta} = 1/3\]corresponding to the angle $70.53^{\circ}$. For the quasi-para position, the answer is obviously $180^{\circ}$, but this can also be checked quickly.
So, the substitent orientation is not quite the same as in benzene, but there are more directions to reach favorable interactions with a target enzyme or a receptor, e.g.
Side note on “three-dimensionality” of molecules
Three-dimensionality of cubane is undisputed because cubane actually encloses a space (that is there is an inside and outside), unlike many other compounds which even if they are rich in stereocenters can still be flattened out without any loss in information about connecitivity, and they are therefore at least topologically two-dimensional. Try out the famous Euler’s formula to convince yourself that: $ V+F-E=2 $ (V: number of vertices, F: number of faces, and E: number of edges) for a 3D body like a cube, but 1 for a planar object like a square or [2.2.2]bicyclooctane. How about adamantane?